Multi-site phosphorylation of yeast Mif2/CENP-C promotes inner kinetochore assembly
Abstract
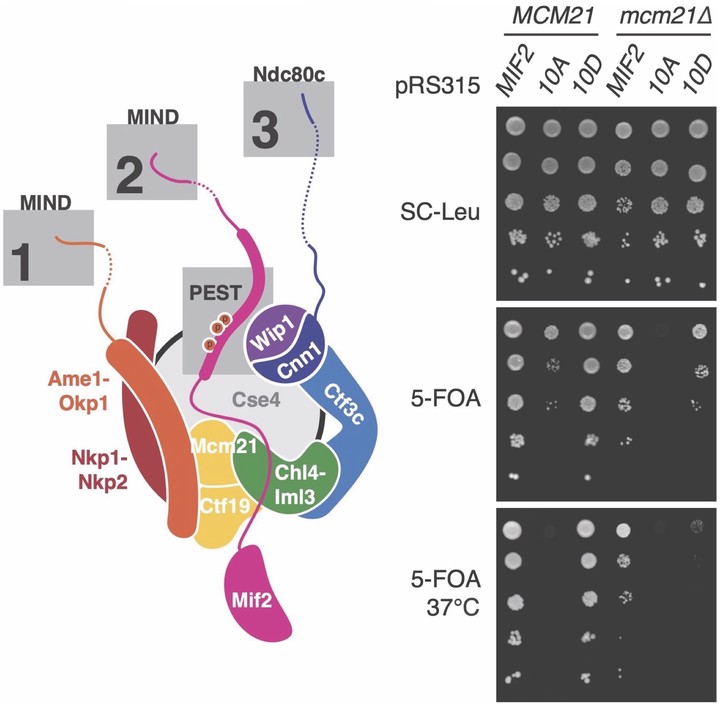
Kinetochores control eukaryotic chromosome segregation by connecting chromosomal centromeres to spindle microtubules. Duplication of centromeric DNA necessitates kinetochore disassembly and subsequent reassembly on the nascent sisters. To search for a regulatory mechanism that controls the earliest steps of kinetochore assembly, we studied Mif2/CENP-C, an essential basal component. We found that Polo-like kinase (Cdc5) and Dbf4-dependent kinase (DDK) phosphorylate the conserved PEST region of Mif2/CENP-C and that this phosphorylation directs inner kinetochore assembly. Mif2 phosphorylation promotes kinetochore assembly in a reconstituted biochemical system, and it strengthens Mif2 localization at centromeres in cells. Disrupting one or more phosphorylation sites in the Mif2-PEST region progressively impairs cellular fitness and sensitizes cells to microtubule poisons. The most severe Mif2-PEST mutations are lethal in cells lacking otherwise non-essential Ctf19 complex factors. These data suggest that multi-site phosphorylation of Mif2/CENP-C is a robust switch that controls inner kinetochore assembly, ensuring accurate chromosome segregation.